Menu
Close
Back
Welcome to
Together is a new resource for anyone affected by pediatric cancer - patients and their parents, family members, and friends.
Learn MoreCOVID-19 stands for coronavirus disease 2019. It is an illness caused by the SARS-CoV-2 virus. COVID-19 affects the respiratory system including the lungs, nose, and throat.
Most people with COVID-19 have mild symptoms, much like a cold or the flu. But some people can develop more serious complications like pneumonia or have problems that are long-lasting.
COVID-19 spreads easily from person to person. People who are infected can spread the virus even if they do not have symptoms.
Staying up to date on your COVID-19 vaccine can help protect against serious illness. Talk to your health care provider about specific vaccine recommendations.
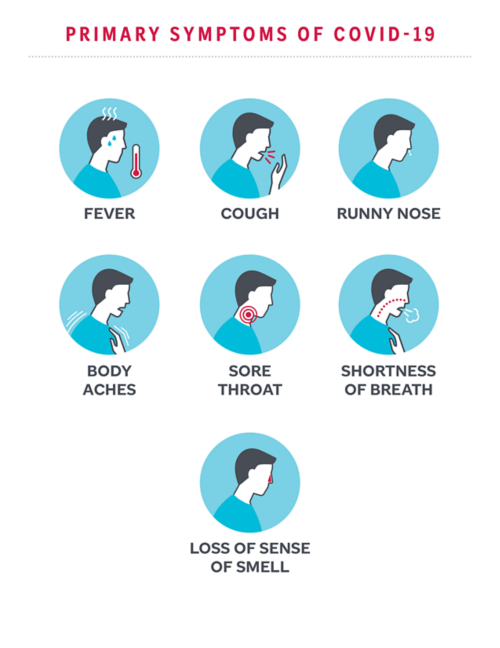
Many symptoms of COVID-19 are similar to those of a cold or the flu. Some people may be infected with the virus and not have any symptoms.
Most people with COVID-19 have mild symptoms. Some people may not show any symptoms. Symptoms of COVID-19 include:
In some cases, people with COVID-19 become very ill. Warning signs of severe illness are:
Children with certain medical conditions or who have weak immune systems are at higher risk for severe COVID-19. Most patients do relatively well if they get COVID-19. But COVID-19 can be life-threatening for some patients.
Some children are at higher risk for serious illness with COVID-19. Conditions or factors that may increase your child’s risk include:
In children with sickle cell disease, COVID-19 can cause:
If your child has a weak immune system, it is important to take steps to prevent illness. Call your doctor if your child has been exposed to COVID-19 or has symptoms.
Wear a mask or face covering as your doctor recommends. If your child is immunocompromised, ask your care team about the type of mask needed and instructions for use.
Simple steps can help prevent the spread of COVID-19 and protect against illness.
The COVID-19 vaccine is a safe, effective way to prevent severe COVID-19. Everyone who is eligible should get vaccinated against COVID-19.
The Centers for Disease Control and Prevention (CDC) recommends that everyone 6 months and older get an updated COVID-19 vaccine.
Side effects of the COVID-19 vaccine are usually mild and include soreness, swelling at the injection site, and body aches. These side effects may occur within the first 3 days of vaccination and resolve within 1–2 days.
If your child has a weak immune system, your health care provider may recommend additional doses of vaccine. Talk with your care team about the vaccine recommendations for your child’s medical needs.

The coloring book is a fun way for children to learn about COVID-19 vaccines and how they work.
View and DownloadGet the latest information on COVID-19 and COVID-19 Vaccines from the Centers for Disease Control and Prevention (CDC).
—
Reviewed: January 2024

Influenza (flu) is a respiratory illness caused by a virus. Some people are at higher risk for flu complications. Learn how to prevent and treat the flu.

Fever is one of the most common symptoms of COVID-19. However, people with COVID-19 may sometimes have a low-grade fever or no fever at all.

Vaccines are an important way to protect against certain illnesses. Learn about immunization schedules and vaccines in children with cancer and other illnesses.