Immunotherapy is a type of cancer treatment that uses the immune system to fight cancer. The immune system is the body’s defense against infection and sickness. It works to attack germs such as bacteria and viruses. The immune system also helps get rid of unhealthy or damaged cells in the body. Because the immune system is a part of the body, immunotherapy is sometimes called biologic therapy or biotherapy.
The basic idea of immunotherapy is simple: help the body defend itself against harmful invaders. However, cancer cells can be tricky. They often find ways to change so that they are hidden from the immune system. They also use several methods to turn off the body’s defense when it tries to attack. In general, immunotherapies work by counteracting some of these different ways that cancers use to escape by:
- Helping the immune system find cancer cells so it can attack them
- Increasing the immune system’s ability to respond to cancer
There are different types of immunotherapy, and they each work in different ways to help the immune response.
Immune cells and immunotherapy: T cells and NK cells
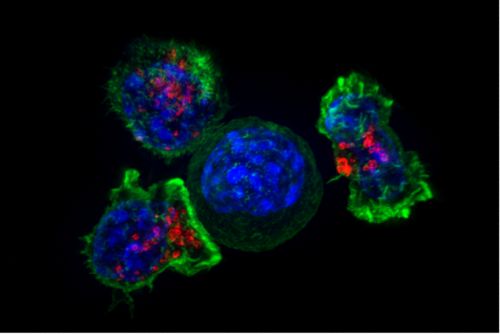
The immune system uses different types of specialized white blood cells, including cells called lymphocytes, to attack germs and other invaders. Two types of lymphocytes, T cells and natural killer (NK) cells, are the main focus of current cancer immunotherapies.
T cells
T cell based immunity is called adaptive, or acquired, immunity because T cells are programmed to attack only after they acquire necessary and specific information about the harmful cell. In other words, for T cells to have an “ok” signal to attack harmful cells, they first need to get information about the cell they’re facing. T cells target cells that they recognize as the body’s own, but that show that something is harming them, such as when a virus infects them. T cells do this by detecting a protein, called an antigen, on the cell’s surface that they recognize as being foreign or harmful. They detect that antigen with a T cell receptor.
Most T cell-based immunotherapies depend on the T cell being able to see the antigen on cancer cell’s surface. Once T cells learn a specific cancer antigen, the body makes many more copies of that T cell that can recognize the antigen so that they can seek out other cancer cells that have that specific antigen and attack them. Some T cells can also remember the antigen and respond again if they see it again. This “memory” is an important way that T cell-based immunotherapies may stop cancer cells with that antigen from coming back (relapse).
Types of T cell based immunotherapies include immune checkpoint inhibitors, vaccines, cytokines, and T cell transfer.
Natural killer cells
Natural killer cells, or NK cells, are another type of lymphocyte important in immunity. NK cells patrol the body looking for cells that they recognize as “not self,” such as bacteria. NK cells do not depend on a specific antigen being present on the cancer cell. NK cell-based immunity, known as innate immunity, is a more general immunity.
Types of NK cell-based immunotherapies include monoclonal antibodies (since they tag the cancer cells and signal NK cells to then come and attack), cytokines, and NK cell transfer.
Types of immunotherapy
Monoclonal antibodies
Monoclonal antibodies are small molecules made in a laboratory that specifically attach to cancer cell antigens. Monoclonal antibodies can work in several different ways. They can be used to:
- Flag cancer cells so that the immune system (NK cells) can find and attack the cells
- Carry toxins or radiaoactive molecules directly to cancer cells to kill them
- Block signals important for cancer cell growth
Because they target specific antigens, treatment with monoclonal antibodies may also be considered a type of targeted therapy.
Immune checkpoint inhibitors
Checkpoint inhibitors are drugs that turn off the “brakes” on the immune system. The immune system has checkpoints or signals that tell it to slow down. These checkpoints are important to keep an immune response from getting out of control and harming healthy cells. Sometimes cancer cells use these signals to hide from the immune system. Checkpoint inhibitors can turn off the “stop” signal so that the immune system can keep going.
Vaccines
Tumor-based vaccines are given to patients that already have cancer. This is different from other vaccines used to prevent disease. The goal of a cancer treatment vaccine is to help the immune system learn what it needs to fight. The vaccine contains a cancer antigen or other tumor marker. This helps train immune cells to attack tumor cells with that marker.
Cytokines
Cytokines are proteins in the body that help regulate the immune system. Interferons and interleukins are specific cytokines that can be used in cancer treatment. These proteins act as signals to stimulate NK cells, T cells, and other immune cells that attack cancer cells.
Adoptive cell therapy
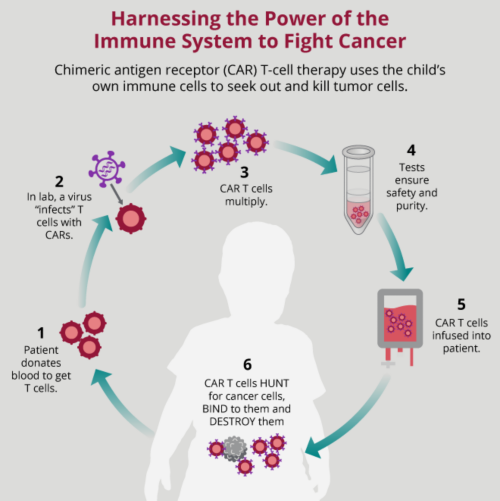
Adoptive cell therapy is a type of immunotherapy that uses donor immune cells or the patient’s own immune cells collected from the blood. In some types of adoptive cell therapy, a patient’s immune cells are changed or engineered in the laboratory to make them better able to attack cancer cells. T cells or NK cells that are not further engineered are mainly used after bone marrow transplantation. These cells are selected from the donor and then given back to patients to destroy tumor cells.
Two engineering approaches are currently used to make tumor-specific T cells. Both approaches follow the same procedure. First, T cells are collected from the patient. Next, in the laboratory, T cells are engineered to make them tumor specific by using a special receptor. These cells are then further grown before they are injected back into patients. After they are infused into the patient, engineered T cells then seek out and destroy cancer cells throughout the body. The tumor-specific receptors are called T-cell receptors (TCR) and chimeric antigen receptors (CAR). There are also studies in progress that explore adding CARs to NK cells.
How is immunotherapy given?
Immunotherapy drugs are usually given by injection through a vein (IV). Immune cells are given by IV or injected directly into the tumor area.
The dose and schedule of treatment depend on the type of therapy. Immunotherapy often involves multiple doses of treatment over a period of time. Treatments may follow a specific schedule, or cycle of treatment and rest. The goal of the schedule is to allow the immunotherapy to work and to give the body a chance to recover.
Doctors monitor patients closely to see how well the therapy works and to watch for side effects.
Side effects of immunotherapy
Side effects of immunotherapy depend on the specific type of treatment, but symptoms are generally a result of immune system activation. Inflammation occurs as the immune system is turned on. This can be a local response or a general response, since immune cells travel to the parts of the body where the “intruder” cells are found. Symptoms are often similar to what happens when the body fights an infection such as a cold or flu. Patients may have fever, chills, body aches, rash, and fatigue.
Other side effects of immunotherapy may include:
- Swelling or weight gain due to immune cell response
- Headaches and confusion
- Change in blood pressure
- Heart palpitations
- Difficulty breathing
- Diarrhea
Future of immunotherapy in childhood cancer
Because immune cells can circulate throughout the body, immunotherapy may also help to kill cancer cells that have spread away from the main tumor. Another potential benefit of using the immune system to treat cancer is that some immune cells can develop a “memory” response. T cells learn to attack specific antigens and can remember that antigen if it comes back. The T cells can then respond more quickly when it sees that type of antigen again. This immune system memory is particularly promising in prevention of cancer relapse.
Immunotherapy is an emerging area of research in childhood cancer. Ongoing clinical trials are studying the use of immunotherapy in several pediatric cancers.